The work done during the process is 30 kJ. Chemistry questions and answers.

100kg Melting Scrap Iron Steel Electric Furnace Electric Furnace Iron Steel Furnace
C_v for nitrogen frackJkg K.

. 23 kg of nitrogen is 2300 g. Find a the volume of the mixture b the partial volumes of the components c the partial pressures of the components d the mole fraction of the components e the specific heats c_p and c of the mixture and f the gas constant of the mixture. O The specific volume of the combined system is double the initial specific volume of one of the vessels.
Civil Engineering questions and answers. The piston is moved compressing nitrogen until the pressure becomes 1 MPa and temperature becomes 160C. Compare your results with the actual value of 1505 kPa.
A 327-m3 tank contains 100 kg of nitrogen at 175 K. Compute the heat added the work done and the change in internal energy if this is done at constant volume. Las herramienta Recortes se está moviendo The pressure of 100 kg of nitrogen N2 at 70C in a 100-m tank is most nearly.
2 1 0 4 K 1 a t m K 7. A 1-m3 rigid tank contains 100 kg R-22 at ambient temperature 15 C. Determine the boundary work done during this process.
Determine the pressure in the tank using a the ideal-gas equation b the van der Waals equation and c the Beattie-Bridgeman equation. Dont type the units. Compare your results with the actual value of 2000 kPa.
21 rows γ m gV ρ g 2 where g acceleration due to gravity units typically ms 2 and value. The pressure of 100 kg of nitrogen N2 at 70C in a 100-m tank is most nearly. Determine the pressure in the tank using a the ideal-gas equation b the van der Waals equation and c the Beattie- Bridgeman equation.
At 0C 32F or 27315K at standard atmospheric pressureIn Imperial or US customary. Determine the pressure in the tank using a the ideal-gas equation b the van der Waals equation and c the BeattieBridgeman equation. Density of nitrogen gas is equal to 1251 kgm³.
For nitrogen C p 1 4 0 0 J k g 1 K 1 and C v 7 4 0 J k g 1 K 1. Substitute values in the above expression. To determine the volume of 23 kg of nitrogen gas we would need to convert the mass of nitrogen to moles and then covert moles to liters at STP.
A 327-m 3 tank contains 100 kg of nitrogen at 225 K. The pressure of 100 kg of nitrogen N2 at 70 degree C in a 100 m3 tank is most nearly. The pressure of 100 kg of nitrogen N2 at 70 degree C in a 100 m3 tank is most nearly.
M3kg 15180C Table A piston-cylinder device initially contains 007 mg of nitrogen gas at 130 kPa and 1200C. QUESTION 4 A pressure vessel contains 8 kg of nitrogen with internal energy of 46 MJ at a pressure of 3 MPa. Engineering Mechanical Engineering QA Library Q6 A 327-m3 tank contains 100 kg of nitrogen at 175 K.
What pressure is a nitrogen cylinder. Hint Use R 297 J kg-K Doctenos. A 327-m3 tank contains 100 kg of nitrogen at 175 K.
2 1 0 4 M According to Henrys law S K P S is the solubility in moles per litre K is the henrys law constant P is the pressure in atm. 05 kg of Helium and 05 kg of nitrogen are mixed at 20 degrees C and at a total pressure of 100 kPa. Nitrogen is now expanded isothermally to a pressure of 100 kPa.
What is the specific internal energy of the gas in MJkg. The pressure of 100 kg of nitrogen N2 at 70C in a 100-m tank is most nearly. Calculate the heat transferred from the nitrogen to the surroundings.
Determine the pressure in the tank using a The ideal-gas equation b The van der Waals equation and c The Beattie-Bridgman equation. We begin be converting the mass to moles. 2 1 0 4 L a.
The nitrogen is now expanded to a pressure of 100 kPa polytropically with a poly- tropic exponent w ue is equal to the speci ratio called isentropic expansion. According to Avogadros Law a gas will always have a volume of 224 L mol at Standard Temperature and Pressure STP. The temperature of 3 kg of nitrogen is raised from 10 o C to 100 o C.
Determine the boundary work done during this process. O The pressure in the combined system is 100 kPa. Compare your results with the actual value of 1505.
2 1 0 4 moles. The properties of nitrogen are R. The nitrogen is now expanded isothermally to a pressure of 100 kPa.
The temperature of 3 kg of nitrogen is r Question The temperature of 3 kg of nitrogen is raised from 10 o C to 100 o C. A 327-m3 tank contains 100 kg of nitrogen at 175 K. 03 kg of nitrogen gas at 100 kPa and 40C is contained in a cylinder.
0 2 7. Hence the solubility S 7. 002 g nitrogen corresponds to 2 8 0.
Compute the difference in work done if heating were at constant pressure and at constant volume. A typical cylinder about 5 tall can hold about 230 cubic feet of nitrogen gas if it is filled to the maximum operating pressure which can be in the range of 2200 psi that is to say if it is full. Compare your results with the actual value of 1505 kPa.
Determine the pressure in the tank using a the ideal-gas equation b the van der Waals equation and Compare your results with the actual value of 1505 kPa. The cylinder will have the maximum allowable pressure stamped on the side near the valve. 1 cubic meter of Nitrogen gas weighs 1251 kilograms kg 1 cubic inch of Nitrogen gas weighs 0000723124 ounce oz Nitrogen gas weighs 0001251 gram per cubic centimeter or 1251 kilogram per cubic meter ie.
Hint Use R 297 J kgK.
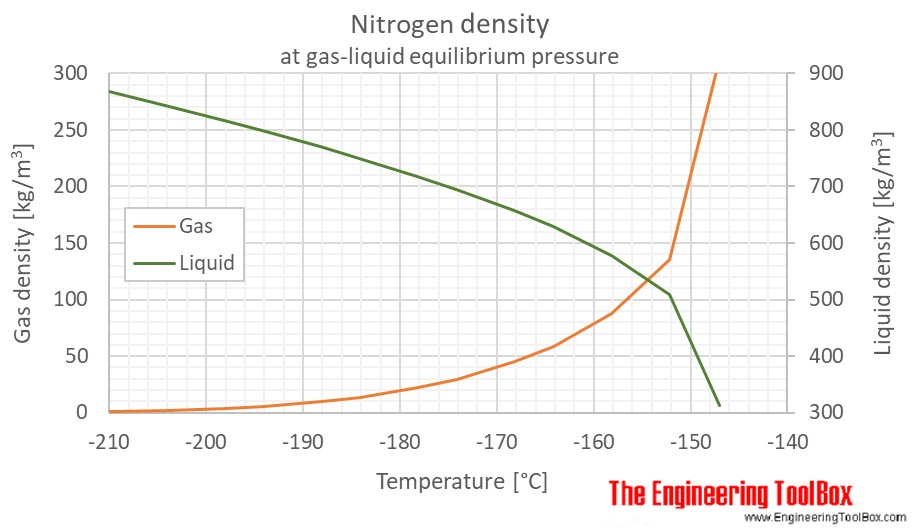
Nitrogen Density And Specific Weight Vs Temperature And Pressure

Thermodynamics Calculate The Work Input Thermodynamics Calculator Relatable

Hydraulic Accumulator Air Cylinder Nitrogen Gas Charging Kit Hammer Device For Hydraulic Breaker Hydraulic Breaker Hydraulic Nitrogen
0 Comments